Vedolizumab is a biological medication used to treat Crohn's disease and ulcerative colitis. Find out more in this article...
Vedolizumab, also known by its brand name Entyvio, is a biological medication that has been approved for use by adults with moderate to severe Crohn’s disease or ulcerative colitis.
It tends to be used to treat people who haven’t responded to other medications (including other biological drugs) or those who are intolerant to them.
Vedolizumab is a biological medication. Biologics are a newer class of drugs available in the treatment of inflammatory bowel disease. They are genetically engineered from living organisms and work by targeting specific proteins and enzymes in your body that cause inflammation.
They are more targeted than some other medications used in the treatment of IBD.
Entyvio is a ‘gut-selective integrin antagonist’ - meaning it only targets the gut and not the rest of the body. It works by binding to a protein known as α4β7 integrin. This protein is attached to a lymphocyte which contributes to inflammation seen in Crohn’s disease and ulcerative colitis. By binding to α4β7 integrin the Entyvio inhibits the lymphocytes in contributing to disease exacerbation.
Vedolizumab has been licensed for use only in adults with moderate to severe Crohn’s disease or ulcerative colitis.
However, some doctors decide to try it on patients under the age of 18 if they are not responding to any other medications.
Before you start taking Entyvio you will have a check-up to ensure it is suitable for you to take. You will be asked questions about any infections you have had, or may currently have, if you’ve had any immunisations, if you have cancer, details of any liver problems or tuberculosis (TB) and if you are pregnant. You will also be asked about any medications you are currently taking.
During the treatment you will receive monitoring.
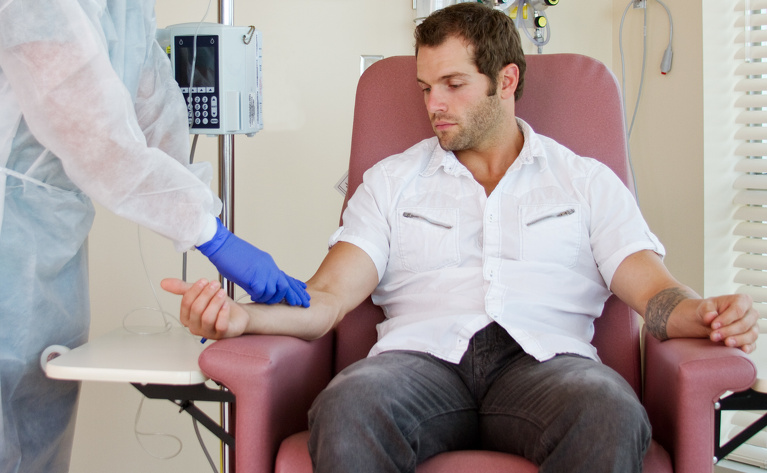
Entyvio is administered through an intravenous (IV) drip into your arm. These infusions usually take place in hospital and are carried out by a specially trained doctor or nurse. Each dose of vedolizumab is usually 300mg. An infusion takes around 30 minutes but your whole appointment may take a couple of hours. You will need to receive regular infusions for the duration that you are on the medication. You will receive the first two doses within two weeks but your doses after that will be spread out and will be received approximately every 6-8 weeks.
Takeda, the company that makes Entyvio, says some people start to see results from the medication in as little as 6 weeks, though it may take up to 14 weeks to have any effect. However, some people do not respond at all to the drug.
It greatly varies from person to person as to whether or not vedolizumab is effective and to what extent.
Your doctor may also prescribe other medication to be taken alongside Entyvio - such as immunosuppressants or steroids.
Your doctor may prescribe you other medication to take at the same as your vedolizumab. If you are taking medications for other conditions you should inform your IBD team so they can check if the medications are known to interact with vedolizumab.
It isn’t currently known what effects vedolizumab can have during pregnancy and current recommendations are not to take it while you are pregnant or while trying to get pregnant. It is also recommended not to get pregnant for 5 months after stopping taking Entyvio.
It also isn’t known if vedolizumab passes into breast milk.
If you are planning on getting, or are pregnant or breastfeeding while taking vedolizumab then you should talk to your IBD team.
Some of the more serious side effects that can happen while taking vedolizumab include:
Some of the more common side effects include: