Adalimumab is a biologic medication used to treat Crohn's disease and ulcerative colitis. Find out more about it in this article...
Adalimumab is a biologic medication that is licensed to be used in the treatment of a variety of medical conditions including moderate to severe Crohn’s disease and ulcerative colitis (both forms of inflammatory bowel disease (IBD).
The original version of adalimumab is called Humira and made by the pharmaceutical company AbbVie, however there are now some biosimilar versions of adalimumab available. Biosimilars are copies of the original medication made by a different company.
Crohn’s disease and ulcerative colitis have been linked to an increase in certain proteins - including one called tumor necrosis factor (TNF). Your body’s immune system naturally produces TNF.
An increase in TNF is linked to an increase in inflammation in the body’s digestive system - which can lead to a worsening of inflammatory bowel disease (IBD) symptoms.
Adalimumab is one of a group of medications which target TNF proteins, bind to them and block them. This helps to prevent inflammation in the body which is hoped will reduce IBD symptoms. The immune system is also suppressed. This can mean that people taking adalimumab are more prone to picking up illnesses and infections.
Adalimumab is licensed in the UK to be used in the treatment for:
Adalimumab is an expensive medication and you may have to apply for funding in the UK from your the NHS locally to be able to receive it. Your doctor will be able to advise you about this. Funding is often done on an annual basis - so there is no guarantee that you will receive funding the following year if you have been granted it this year.
Before taking adalimumab for Crohn's disease or ulcerative colitis you should tell your doctor all of your symptoms and if you believe you may currently have any infections.
You should also tell your doctor if you are on any other medications, over-the-counter medicines, vitamins or supplements.
Humira cannot be taken at the same time as:
You should also especially inform your doctor if you are on
If you are considered eligible to take adalimumab your doctor will carry out some tests. This include tests for tuberculosis (TB) and hepatitis B. If these tests come back clear and your doctor has no other concerns then you may be eligible for adalimumab.
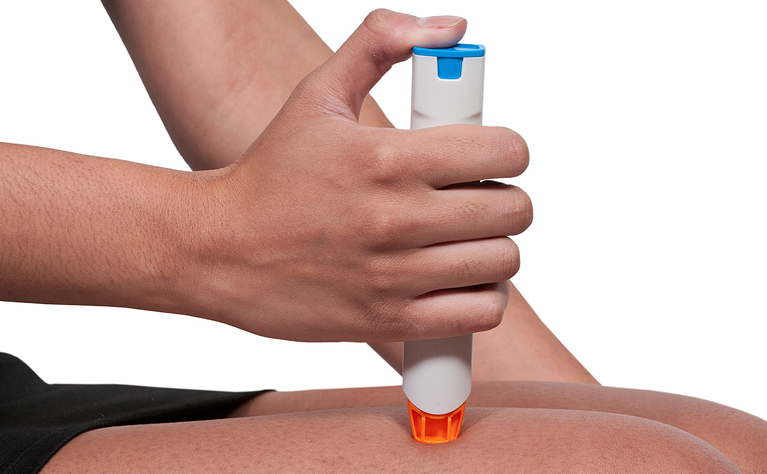
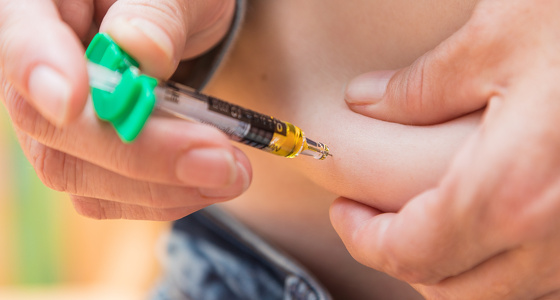
Adalimumab is administered by subcutaneous injection at regular intervals (generally every other week). Subcutaneous means that an injection is given into the fat between your skin and muscle.
Some people administer the injection themselves at home, while others receive it from a nurse. If you are considered suitable to self-administer you will receive full training from a qualified professional who will teach you how to do it and observe you the first couple of times. If you are self-administering adalimumab your dosages will be delivered to you in loaded pens with a short needle under the cap. These pens need to be kept refrigerated.
The first time you receive adalimumab will be your ‘loading dose’. This will be a higher dose than the usual dose you will receive. The ‘loading dose’ is usually 160mg of adalimumab - four loaded pens. The second dose is taken 2 weeks later - usually 80mg or 2 loaded pens. After that you will usually take 40mg (one loaded pen) every two weeks.
If you are self-administering your adalimumab you will be provided with a sharps bin to discard of the used pen.
Some people report that adalimumab stings when it is administered.
Clinical studies have shown that some people start to see results within 4 weeks but most people see them by 8 weeks. If you haven’t started to notice any difference to your symptoms after 12 weeks then you should contact your doctor, although you should receive regular monitoring from your doctor via appointments and/or blood tests.
Some of the serious side effects of taking adalimumab include:
It’s important that if you notice any new symptoms while taking adalimumab that you contact your doctor or care team to inform them.
Common side effects of adalimumab include:
There is an increased chance of getting lymphoma or other cancers in people who take TNF blockers such as adalimumab.
adalimumab needs to be kept refrigerated and protected from light until it is used. It can be stored up to temperatures of 25c (for example when travelling) for up to 14 days as long as it is protected from light. Once it has been kept out of the fridge it must be used or discarded within 14 days of being removed.
Adalimumab does interact with some other medications so it’s important that before you start taking it you give a full list of medications to your doctor. It cannot be taken at the same time as:
Some people use adalimumab alongside other medications for their IBD such as mesalazine, methotrexate, steroids and azathioprine. Your doctor will advise you as to whether it is safe for you to take other medications at the same time.