Biologics are the newest medication to be used to treat Crohn's disease and ulcerative colitis. Find out more about them in this article.
Biologics are medicines genetically engineered from living cells. They target certain proteins and enzymes in your body involved in causing inflammation. This is different to some other inflammatory bowel disease (IBD) medicines which suppress your whole immune system, such as azathioprine, 6-mercaptopurine and steroids.
Biologic medicines are relatively new, but have been shown to be effective in treating and maintaining remission in people with moderate to severe Crohn’s disease and ulcerative colitis. They may be an option when other medicines, such as immunosuppressants (such as azathioprine, 6-mercaptopurine) and steroids haven’t been effective.
However, biologics don’t work for everyone and can also stop working after you’ve been taking them for a while.
There are several different categories of biologic medicines that all work in different ways.
Anti-TNF medicines target and bind to a protein called tumour necrosis factor-alpha (TNF-alpha). This reduces inflammation and helps improve your symptoms. The anti-TNF medications used in Crohn's disease and ulcerative colitis are:
Anti-interleukin medicines target and bind to proteins interleukin-12 and interleukin-23, stopping them working. This helps reduce inflammation and relieve your symptoms. There is one anti0interleukin medication used in IBD:
Ustekinumab - brand: Stelara
Anti-integrin medicines stop white blood cells entering the lining of your gut. Your immune system produces white blood cells to fight infection and in IBD your body produces too many white blood cells, causing inflammation. These medications only affect the immune system in your gut, which can mean you have fewer side effects. There is one anti-integrin medication used in Crohn's disease and ulcerative colitis:
Small molecule drugs are compounds that regulate or modify a biological process. They are used as an anti-inflammatory medicine that modifies the immune system but in a different way to biologics. They affect the immune system by blocking the ability of lymphocytes (white blood cells) to escape from the lymph nodes into the gut, where they can cause inflammation. They achieve this by sticking to the lymphocytes which stops them travelling through the gut mucosa, the innermost layer of the gastrointestinal tract.
The new small molecule medications approved for use in IBD are possible treatment options for adults with moderately or severely active ulcerative colitis when other treatments have been tried but prove unsuccessful. This means, in general, if your ulcerative colitis did not respond adequately to standard treatments or biologic treatments, you have not got better or have stopped getting better while on them, or you can’t tolerate standard treatments, biologic treatments and the side effects.
The next generation of small molecule medications block enzymes involved in activating your body's immune system, reducing inflammation. They are known as Janus kinase (JAK) inhibitors. The JAK inhibitor medications used in treating ulcerative colitis are:
Other new small molecule medications belong to a class of medicines called sphingosine 1-phosphate (S1P) receptor modulators. The sphingosine 1-phosphate (S1P) receptor modulator used in treating ulcerative colitis is:
Sometimes you will be prescribed a biosimilar. Biosimilars are very close copies of the original brand of biologic medicine (known as the originator). They are thoroughly tested to make sure they are as safe and effective, and work in the same way, as the originator.
Other companies can start making copies of the original medicine when it comes off patent. So far this has happened with infliximab and adalimumab.
All medicines come with benefits and potential risks and some people experience serious side effects when taking biologics. They have been thoroughly tested on humans, and continue to be tested, along with an adverse reactions to them being recorded. A recent study on 185 paediatric IBD patients found that there was a good level of general safety in the use of biologic treatment for children with IBD. The study collected information about immediate and delayed adverse events (side effects) and whether these events resulted in biologic treatment being stopped. The main issue arising from the study was reactions to infliximab infusions1.
Any decision to start biologic treatment will be made after you and your IBD team have discussed all of these benefits and risks involved. Many factors will be taken into account, including how active your disease is, what other conditions you may have, other medication you may be taking, and which method of taking medicine suits you best.
Starting a new medicine can feel a little overwhelming, especially when you learn it might involve regular injections or infusions in hospital. Remember that your IBD team will have weighed up all the risks and benefits of starting you on a biologic medicine, with the aim of getting you well as quickly as possible.
Before starting your treatment, you will have checks to make sure biologics are right for you.
You will have a blood test that will check:
You may also have a chest X-ray to check you don’t have tuberculosis.
Your IBD team will need to know if you:
Until recently, biologic medicines were not able to be taken orally, as they are destroyed by your stomach acid and enzymes, and are also poorly absorbed by your digestive system2.
However, in the past couple of years a new type of biologic medicine that can be taken orally has been approved for use in moderate to severe ulcerative colitis. Tofacitinib and figotinib are now options for patients who find other medicines and biologics do not work effectively, have stopped working, or give side effects that are too difficult to cope with.
Oral biologics are tablets which you usually take twice a day, one tablet in the morning and one in the evening.
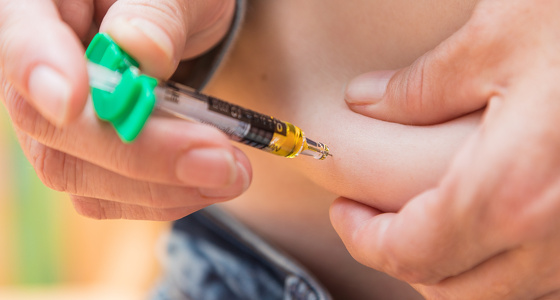
Some biologic medicines are given as an injection under your skin. At first, a doctor or nurse will give you the injections, however once you are used to having your medicine this way, they will teach you how to inject yourself. This can be a daunting thought, but you won’t be left to do it alone until you are ready and capable. Some people prefer to have a family member or friend trained to give the injection instead.
The biologic medicines come as ready to use prefilled syringes or prefilled injection pens, which will be delivered to your home and must be stored in your fridge until you need them. You will also be given a sharps bin to safely dispose of used items.
How often you have injections will depend on your condition, and which biologic you are taking.
Find out more about having subcutaneous biologic injections
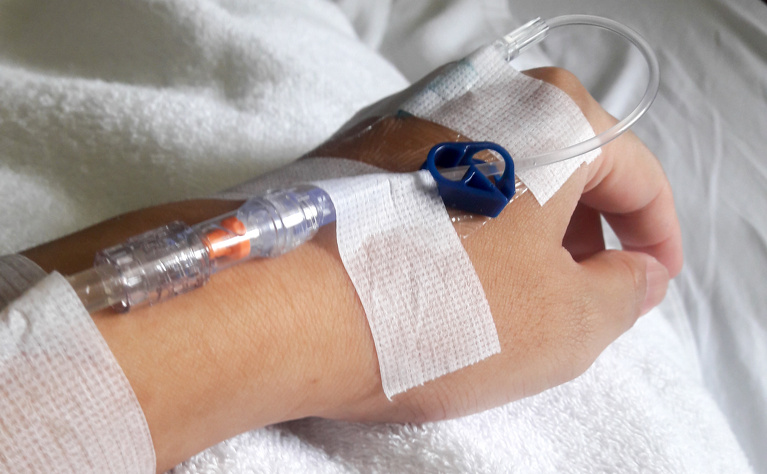
Some biologic medicines are given as an infusion. This means that a cannula (a very thin flexible tube) is put into a vein in your arm, and the medicine goes through a drip straight into your bloodstream. Infusions are usually given by a nurse or doctor in hospital and you will be a day patient - you won’t need to undress, get into a bed or stay overnight.
At the beginning, the infusion process can take several hours, however it might take less time after a few successful treatments.
Again, how often you have infusions will depend on your condition and which biologic you are taking.
All biologic medicines have the potential risk of side effects. Some side effects can happen straight away, as a direct response to the medicine. This can include allergic reactions, which can be very serious and require immediate medical assistance. You will be monitored closely after your initial injections and infusions to check for this type of reaction.
Some side effects may appear gradually, or after a period of time. Some can be mild and will go away on their own, and some can be serious and may require treatment. Sometimes side effects can result in you having to stop a medicine, or change to a different one.
Some people will experience no side effects at all.
Different biologic medicines have the potential for different side effects. Your IBD team will give you detailed information about the particular medicine you are taking, and you can also read the patient information leaflet included in every medicine packet. More details about the specific side effects for each medication are available in the following articles...
Everyone responds differently to different types of biologic medicines. Some patients report feeling better as soon as a few days after their first treatment. It can however take a number of weeks for your treatment to have a noticeable effect.
Biologic medicines don’t work for everyone. You will be monitored regularly by your IBD team to review how well you are responding to your biologic medicine. If you don’t respond within a certain period of time, you may be able to try a different biologic medicine to see if it works better for you.
Sometimes a biologic medicine which has been working for you will start to become less effective, and you may become unwell. This can be caused by your immune system developing antibodies to the biologic, reducing its effectiveness. If this happens, you may be able to try a different biologic instead. Sometimes immunosuppressant medicines such as azathioprine or methotrexate are prescribed in combination with biologics to reduce the risk of these antibodies being produced.
If a biologic medicine works for you, you may be on it for a long time. You will be reassessed regularly to check that it is still the right treatment for you. Sometimes, if you have been in remission for a year or more, it may be decided that you can stop taking your biologic medicine. If you then become unwell again, you should usually be able to start your biologic treatment again.