Stelara (ustekinumab) is a biologic medication approved to be used for the treatment of moderate to severe Crohn’s disease and ulcerative colitis in adults. Find out more about it in this article.
Stelara is the brand name for ustekinumab. It is a biologic medication made by the pharmaceutical company Janssen that is approved to be used for the treatment of a variety of medical conditions including moderate to severe Crohn’s disease and ulcerative colitis in adults.
It is often used to treat people who haven’t responded to other medications (including other biological drugs) or those who are intolerant to them.
Crohn’s disease and ulcerative colitis have been linked to an increase in certain proteins - including two called interleukin-12 and interleukin-23. Interleukins are naturally produced in the body.
The interleukins are linked to the stimulation of immune responses in the body, including inflammation - which can lead to a worsening of inflammatory bowel disease (IBD) symptoms.
Stelara is one of a group of medications called interleukin inhibitors - it targets and binds to the interleukin proteins, preventing them from working. This helps lessen inflammation in the body which will hopefully reduce IBD symptoms. The immune system is also suppressed. This can mean that people taking Stelara are more prone to picking up illnesses and infections.
Stelara has been approved for use by:
Adults with Crohn’s disease
Adults with ulcerative colitis
You shouldn’t take Stelara if you have ever had an allergic reaction to ustekinumab or any of its ingredients. This includes latex, which is present in the pre-filled syringes used to deliver Stelara. If you have a latex allergy then you should speak to your doctor as vials which do not contain latex can be provided.
You should tell your doctor if you have any history of tuberculosis (TB) or any exposure to people with TB. You should not be given Stelara if you have active TB. If you have underlying, inactive TB, this will need to be treated before starting Stelara.
Before taking Stelara you should tell your doctor all of your symptoms and if you believe you may currently have any infections, are pregnant, are planning to get pregnant or are breastfeeding.
Tell your doctor if you are taking, or have recently taken any other medications, over-the-counter medicines, vitamins or supplements.
You should also tell your doctor if you have recently had, or are due to have any vaccinations.
The first dose of ustekinumab is delivered via intravenous infusion (a needle placed in a vein in your arm). You will need to attend a hospital or medical centre to receive this. The initial infusion will last around an hour.
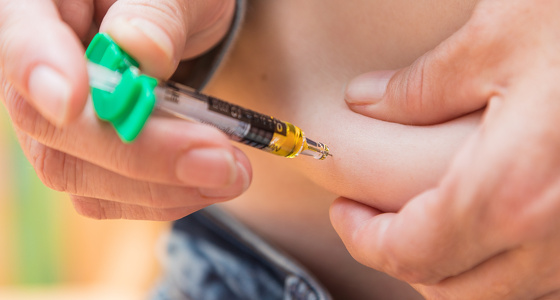
The next dose is administered by subcutaneous injection eight weeks later. Subcutaneous means that an injection is given into the fat between your skin and muscle. After this you will have injections approximately every eight or 12 weeks, depending on what your doctor decides.
Some people administer their injections themselves at home, while others receive them from a nurse. If you are considered suitable to self-administer you will receive full training from a qualified professional who will teach you how to do it and observe you the first couple of times.
If you are self-administering your Stelara, in most parts of the UK it can be delivered to your home, and should be kept in the fridge (see section below on how to store). If not available for delivery you may need to collect from your hospital. You will be provided with a sharps bin to safely dispose of used syringes.
Some patients report pain and redness at the injection site. You can take your medication out of the fridge 15-30 minutes before you administer it which may feel more comfortable. Do not leave it out of the fridge for more than 30 minutes. You can try applying an ice pack for a couple of minutes to the injection site just before you inject.
Everyone responds differently when taking a new medicine, and Stelara doesn’t work for everyone. Clinical trials have shown that some patients start to see results within three weeks in Crohn's disease and two weeks in ulcerative colitis, but most people who respond to Stelara see an improvement within six weeks. In some people it could take longer. You may be taken off Stelara if you have any serious side effects or if you have not responded within 16 weeks of starting your treatment.
Once your treatment has started you will be monitored regularly to check whether ustekinumab is working for you. As your immune system is weakened, you will also be monitored for signs of infection.
Your IBD team will tell you what checks you need and how often. It is important that you tell your doctor about any new symptoms you notice. This will help prevent any potential complications or catch them at an early stage.
Your IBD team should give you a check-up to see whether you should continue having Stelara after 12 months of treatment.
Some of the immediate side effects of ustekinumab include:
These symptoms could indicate that you are having an allergic reaction to Stelara. If you notice any of these immediately after administering ustekinumab, or in the days following an infusion or injection, contact your doctor or care team to inform them.
Serious side effects of taking Stelara include:
Common side effects of Stelara include:
Stelara weakens the immune system and can make you more prone to infections. The following symptoms may be a sign of infection. Tell your doctor if you experience:
Less commonly, some patients taking Stelara may develop tooth infections, a blocked nose, depression, bleeding or bruising at the injection site, temporary muscle weakness in the face, yeast and urinary tract infections.
As ustekinumab suppresses the immune system, it theoretically increases your chance of developing some cancers.
Notify your doctor or care team of any new symptoms you develop whilst taking Stelara.
You should try to keep the pre-filled syringes refrigerated (between 2-8°C). They can be stored outside the fridge up to temperatures of 30°C for up to 30 days, but it must be used within this 30 day period.
Most other medications can be taken safely whilst on Stelara. Your doctor will advise you if it is safe to take other medications at the same time as Stelara. It’s important that you provide a full list of the medications, including non-prescribed, you are taking, or have taken recently, especially if those medications affect your immune system.
Some patients will be prescribed other medications alongside ustekinumab, such as azathioprine, methotrexate and steroids.