Combination therapy, taking two treatments at the same time, is sometimes used in IBD. Find out more about the combination therapy of biologics and immunomodulators in this article.
Combination therapy means using two or more different therapies (treatments) at the same time to try to improve the outcomes for the patient. Monotherapy is when a single therapy (medicine) is used.
In inflammatory bowel disease (IBD) combination therapy usually means using a biologic medication at the same time as an immunosuppressant, such as azathioprine or methotrexate. The most common combination therapy, and the one that has been most researched, is an anti-TNF alpha medication (such as infliximab or adalimumab) combined with azathioprine. This article mostly focuses on this form of combination therapy.
Some of the reasons why combination therapy with biologics and immunosuppressants may be beneficial include:
However, not all studies into combination therapy have shown the benefits above, particularly those looking at newer biologics (such as vedolizumab and ustekinumab) where monotherapy may be superior. The risks of taking an additional medicine in combination therapy also need to be considered.
The pharmaceutical companies producing infliximab, adalimumab, vedolizumab and ustekinumab have all run trials introducing their biologic to patients who haven’t been doing well on immunosuppressants alone. While still taking the immunosuppressant they were also placed on a biologic medication to see if it improved their IBD. Although the results didn’t necessarily show that these patients did any better on the combination therapy than those taking just a biologic, the studies did show that these patients had high biologic drug levels in their body and the anti-drug antibody levels were lower1. The reason many biologics stop working for some Crohn’s disease and ulcerative colitis patients is because they build up too many anti-drug antibodies, so taking an immunosuppressant at the same time may help with this.
A study in 2010 (called the SONIC study) of IBD patients who had never taken an immunosuppressant and a biologic before found that patients receiving combination therapy with infliximab and azathioprine had better clinical and endoscopic outcomes compared to patients who were receiving only infliximab2. This study paved the way for adult gastroenterologists to routinely start using combination therapy.
Later reanalysis of the data from this study found that clinical outcomes were the same in patients who had the same drug levels of infliximab in their body, whether or not they were taking azathioprine as well. This suggests the azathioprine may not improve the efficacy of the biologic, but how well the body maintains levels of it. Through maintaining higher levels of the biologic drug a lower dose of the biologic could be given.
More recently a study looking at combination therapy in newer biologics ustekinumab and vedolizumab found clinical outcomes were no better after one year in those who took the biologic alongside an immunomodulator than in those who took just the biologic3. The researchers did consider that this difference in results between the older TNF-alpha inhibitors (infliximab and adalimumab) and the newer biologics could be because antibodies appear to form more slowly against the new biologics, meaning there could be higher levels of the drug in the patient’s blood4.
It is usually used in patients who have moderate to severe Crohn’s disease or ulcerative colitis which isn’t responding to single medications.
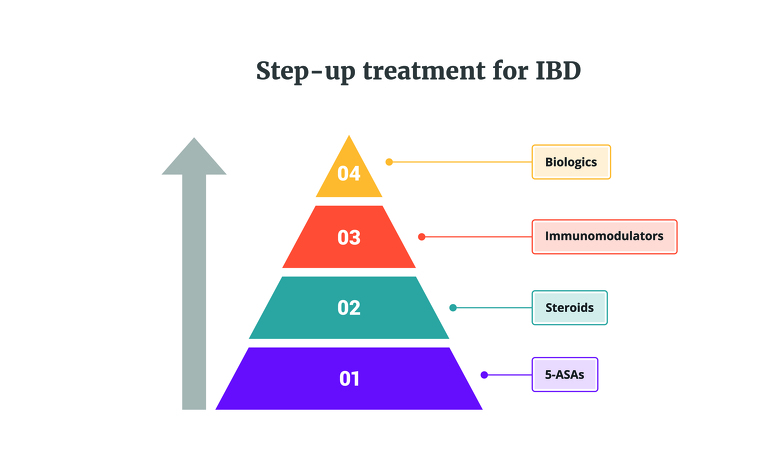
Traditionally treatment of IBD follows a step-up approach, working through a pyramid of different medications until an effective treatment is found for the patient’s Crohn’s disease or ulcerative colitis. Biologics are at the top of this pyramid, with immunosuppressants on the step below. Due to following this treatment pathway, patients who start taking a biologic treatment are usually already taking an immunosuppressant medication which they haven’t been responding well to. This can then result in combination therapy, with the patient starting a biologic while continuing to take the immunosuppressant. A patient may stay on the immunosuppressant for six to 12 months before stopping it1.
However, in some cases the step-up treatment pathway isn’t followed and a doctor makes a decision on a case-by-case basis to follow a different route, perhaps starting a patient straight on a biologic as their first treatment.
In an article in the Gastroenterology & Hepatology Journal in April 2012 Dr Jean-Frederic Colombel speaking about combination therapy in Crohn’s disease patients (CD), said: “The patients in whom I use combination therapy very early after diagnosis are patients with diffuse extensive disease involving the small bowel and colon; patients with disease that involves the upper gastrointestinal tract; patients with complex perianal fistulizing disease, who often present with complications of CD at diagnosis; patients with very severe endoscopic lesions that predict a higher risk of surgery; and pediatric CD patients who are at risk of growth problems.”5
He continued: “The efficacy of combination therapy offers a huge potential benefit, and use of combination therapy is most advantageous in patients with the most severe disease. In selecting a treatment for a specific patient, however, clinicians must consider the risk‑benefit ratio for that individual.”
Ultimately, whether to use combination therapy will be a decision between your IBD team and you. Your doctor will look at your individual circumstances and balance this up alongside the latest research, along with the risks of taking an additional medication.
Studies have looked at stopping either the biologic or the immunosuppressant in IBD patients who are in clinical remission (their IBD is under control). Around 50% of patients who stop one of the medications used in combination therapy stay well for several years when in deep remission1. Being in deep remission (with tests showing this) is key to achieve this longer lasting remission.
Whether you stop taking one or both medications if you are in remission will be something you and your doctor will discuss, weighing up the risks and benefits.
Biologic medications and immunosuppressants each come with their own risks and side effects, but so does leaving your IBD untreated. The question is whether when these two medications are combined the risks increase.
Researchers have found that one of the biggest risks when taking combination therapy of a biologic and an immunosuppressant is an increased risk of contracting infections6.
There has also been concerns about an increased risk of lymphoma, a type of cancer, when taking thiopurines (such as azathioprine). Despite research, the exact risks of taking thiopurines and lymphoma are still not clear cut. A 2013 study7 found the incidence rates of lymphoma were:
At particular risk are men, patients younger than 30 and patients older than 50.
Later a literature review, published in 20208 found that anti-TNFs can contribute to an increased risk of lymphoma and when thiopurines and anti-TNFs are used in combination this risk is then further increased.
If you are considering treatment with anti-TNF biologics or thiopurines, or a combination of these, research such as this can sound scary. However it’s important to know that in its conclusion the study does say: “The absolute risk of lymphoma is low and should not prevent the use of thiopurines and anti‐TNF agents, either alone or in combination, when they are needed.”
You also need to remember that leaving your IBD untreated could lead to many complications.
One way to reduce the risks of combination therapy could be to use the lowest dose possible of the medications.
A study reviewing 99 patients on combination therapy of azathioprine and infliximab or adalimumab found that there were no differences in the number of antibodies formed and in inflammatory biomarkers between those on a low dose of azathioprine (less than 2mg per kg of weight) and a standard dose of azathioprine (more than or equal to 2mg per kg of weight)9. This suggests that the lowest possible dose of azathioprine in combination therapy is appropriate and keeping the dose low helps to mitigate against some of the risks of taking this immunomodulator.
As covered earlier in this article combination therapy of biologics and immunomodulators may not be needed when taking ustekinumab or vedolizumab, however more studies into this are needed.
At the moment data on the effectiveness and safety of taking biologics and immunosuppressants in children with Crohn’s disease and ulcerative colitis is more limited10.
Due to this lack of research the use of combination therapy in paediatric patients with IBD is left to the treating doctor to balance, with a suggestion of withdrawing the immunomodulator after six months if optimum therapeutic drug levels of this drug have been reached11.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5479343/.
https://www.nejm.org/doi/full/10.1056/nejmoa0904492
https://www.cghjournal.org/article/S1542-3565(20)30973-3/fulltext
https://www.medpagetoday.com/gastroenterology/inflammatoryboweldisease/87553
https://onlinelibrary.wiley.com/doi/10.1111/apt.16050
https://academic.oup.com/ecco-jcc/article/14/Supplement_1/S468/5705894
https://www.naspghan.org/files/The_Role_of_Combination_Therapy_in_Pediatric.34.pdf
https://www.gastroenterologyandhepatology.net/archives/august-2020/positioning-biologic-therapies-in-the-management-of-pediatric-inflammatory-bowel-disease/